giugno 03, 2020 - Philips
FDA issues Emergency Use Authorization for Philips’ new acute care patient monitoring solutions for hospital-based remote monitoring
Comunicato Stampa disponibile solo in lingua originale.
- Philips’ IntelliVue Patient Monitors MX750/MX850, IntelliVue 4-Slot Module Rack FMX-4 and IntelliVue Active Displays AD75/AD85 receive FDA Emergency Use Authorization for use in the US during the COVID-19 emergency [1]
- New acute care patient monitoring solution offers an expanded, real-time view of a patient’s vital signs for bedside and remote monitoring in the hospital
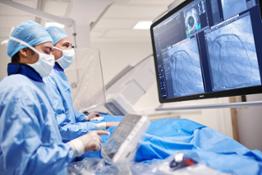
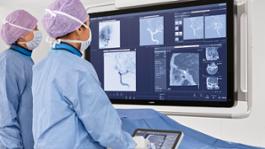
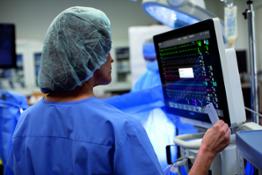
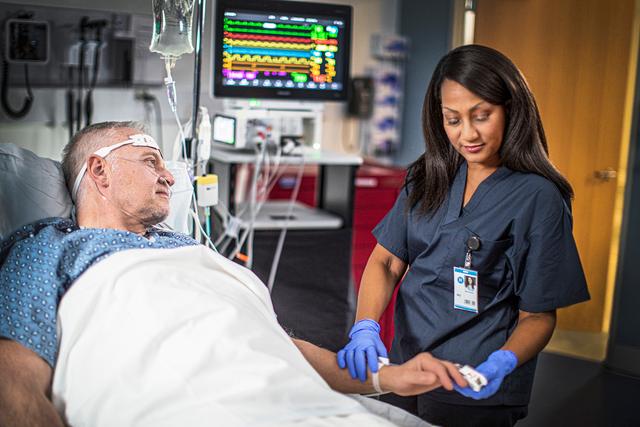
Amsterdam, The Netherlands - Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in #health technology, today announced that the US FDA has issued an Emergency Use Authorization (EUA) for Philips’ IntelliVue Patient Monitors MX750/MX850 and its IntelliVue Active Displays AD75/AD85, for use in the US during the COVID-19 #health emergency [1]. These patient monitoring solutions support infection-control protocols and remotely provide critical patient information for caregivers, capabilities that are much needed when caring for hospitalized COVID-19 patients.
Philips’ IntelliVue Patient Monitors MX750/MX850 and IntelliVue Active Displays AD75/AD85 received CE mark in 2019 and are already being used in hospitals across Europe. The EUA allows #philips to start delivering the new remote patient monitoring solution to hospitals in the US, and the company is committed to submitting a 510(k) to FDA for this acute care solution in the course of 2020.
As countries across the globe continue to combat COVID-19, while gradually resuming elective care, #philips is significantly increasing its patient monitor production to address the demand for increased Intensive Care Unit (ICU) capacity. Hospitals and #health systems are increasingly relying on #health technology to better manage the influx of COVID-19 patients they are seeing in Emergency Rooms (ERs) and ICUs. To minimize staff exposure to the virus that causes COVID-19 while still delivering quality care, there is a critical need for patient monitors that enable clinicians to remotely monitor a patient’s condition.
“As the world continues to battle against COVID-19, we’re committed to ramping up production of all critical solutions that can help in this time of crisis,” said Peter Ziese, General Manager, Monitoring and Analytics at #philips. “This FDA EUA for our MX750 and MX850 monitors and IntelliVue AD75 and AD85 Active Displays allows us to do that for these remote patient monitoring solutions, which are of vital need in the ICU. At #philips, being able to provide the right information at the right time to caregivers has always been a top priority. Now more than ever, there’s an urgent need to make sure those on the frontline have all the available resources at their disposal.”
The IntelliVue Patient Monitors MX750/MX850 and IntelliVue Active Displays AD75/AD85 offer advanced functionality and clinical decision support capabilities such as Philips’ IntelliVue Horizon Trends information view, which shows deviations in vital signs (for example, CO2 and heart rate) to contextualize a patient’s condition, while also supporting infection-control protocols and access to key information both remotely and at the bedside. Features such as Philips’ Alarm Advisor and Alarm Reporting help to reduce alarm fatigue for caregivers, while the smooth glass surfaces, rounded edges and special surface material of the monitors and displays facilitates cleaning and disinfection.
The MX750 and MX850 monitors are the latest additions to Philips’ portfolio of integrated patient monitoring solutions to support improved clinical and operational workflows. The MX750 and MX850 also include updated features, including enhancements to monitor and assess clinical and network device performance, and additional functionalities to strengthen cybersecurity.
For more information on how #philips is addressing COVID-19 globally, please visit the Philips centralized COVID-19 hub.
[1] The status of the Emergency Use Authorization can be found on the FDA website.
- The IntelliVue Patient Monitors MX750/MX850, IntelliVue 4-Slot Module Rack FMX-4 and IntelliVue Active Displays AD75/AD85 have neither been FDA cleared or approved for the indication to assist in for monitoring and recording of, and for generating alarms for, multiple physiological parameters of adult, pediatric, and neonate patients having or suspected of having Coronavirus-2019 (COVID-19)
- The IntelliVue Patient Monitors MX750/MX850, IntelliVue 4-Slot Module Rack FMX-4 and IntelliVue Active Displays AD75/AD85 have been authorized for the above emergency use by FDA under an EUA
- The IntelliVue Patient Monitors MX750/MX850, IntelliVue 4-Slot Module Rack FMX-4 and IntelliVue Active Displays AD75/AD85 have been authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of medical devices under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.











 Inglese
Inglese  Condividi
Condividi Condividi via mail
Condividi via mail  Automotive
Automotive Sport
Sport Events
Events Art&Culture
Art&Culture Design
Design Fashion&Beauty
Fashion&Beauty Food&Hospitality
Food&Hospitality Tecnologia
Tecnologia Nautica
Nautica Racing
Racing Excellence
Excellence Corporate
Corporate OffBeat
OffBeat Green
Green Gift
Gift Pop
Pop Heritage
Heritage Entertainment
Entertainment Health & Wellness
Health & Wellness